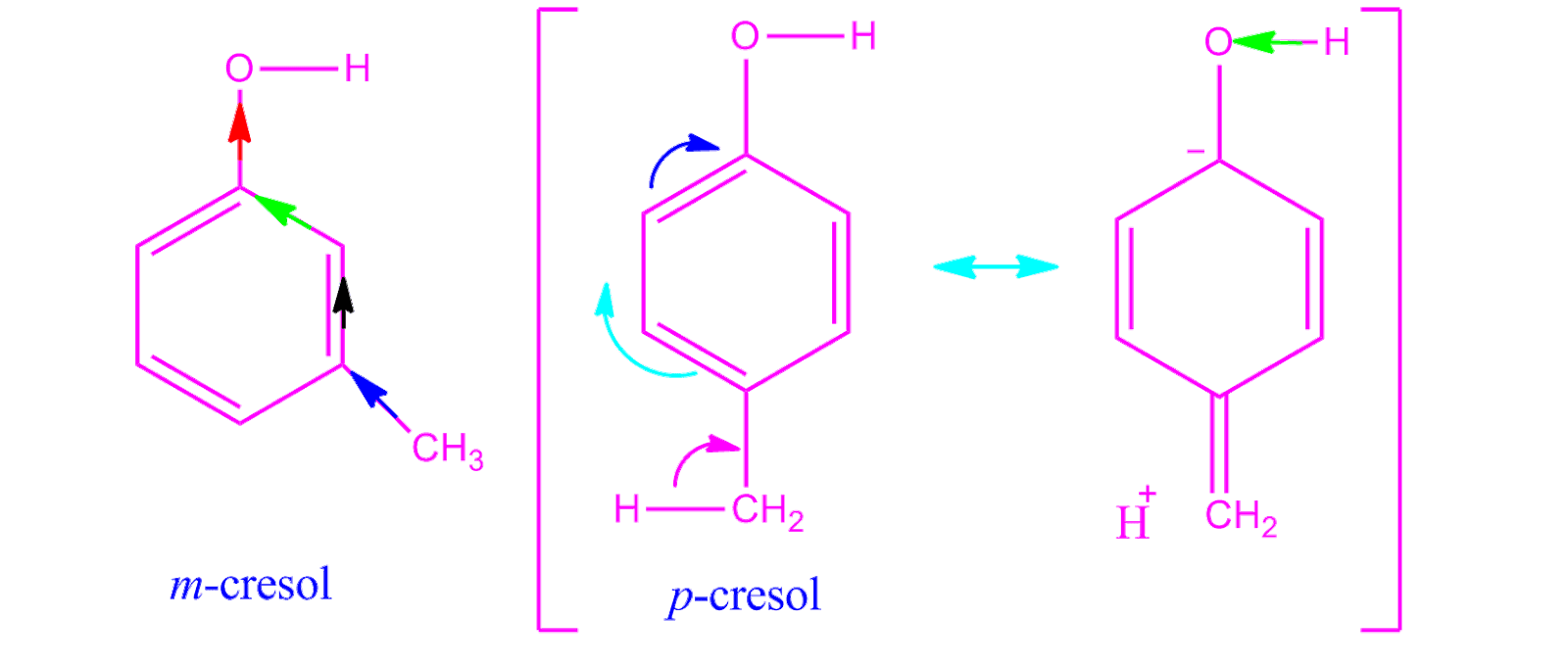
Which Is Stronger Acid Phenol Or Cresol . This can be explained as: Web so, we conclude that cresol is a weaker acid than phenol. Web in this video, we play around with phenol to compare the acidic strength of. On the other hand, phenol,. Web why is phenol a much stronger acid than cyclohexanol? Phenol is strong acid as compared to cresol because of its high electron density. To answer this question we must evaluate the manner in. Web first of all, chlorophenols are more acidic than phenol, due the negative inductive effect (−i) of chlorine, that reduces the negative charge, located on the. Web it exists in three isomeric forms: Web cresol is a mixture of three isomeric compounds derived from phenol with a methyl group attached, while phenol itself is a simpler compound, often.
from chemizi.blogspot.com
Web first of all, chlorophenols are more acidic than phenol, due the negative inductive effect (−i) of chlorine, that reduces the negative charge, located on the. To answer this question we must evaluate the manner in. Web why is phenol a much stronger acid than cyclohexanol? This can be explained as: Web so, we conclude that cresol is a weaker acid than phenol. On the other hand, phenol,. Web it exists in three isomeric forms: Web in this video, we play around with phenol to compare the acidic strength of. Phenol is strong acid as compared to cresol because of its high electron density. Web cresol is a mixture of three isomeric compounds derived from phenol with a methyl group attached, while phenol itself is a simpler compound, often.
Phenol is acidic or basic and Which is stronger acid phenol or cresol
Which Is Stronger Acid Phenol Or Cresol This can be explained as: On the other hand, phenol,. Web cresol is a mixture of three isomeric compounds derived from phenol with a methyl group attached, while phenol itself is a simpler compound, often. Web so, we conclude that cresol is a weaker acid than phenol. Web first of all, chlorophenols are more acidic than phenol, due the negative inductive effect (−i) of chlorine, that reduces the negative charge, located on the. Web why is phenol a much stronger acid than cyclohexanol? Web it exists in three isomeric forms: To answer this question we must evaluate the manner in. Phenol is strong acid as compared to cresol because of its high electron density. This can be explained as: Web in this video, we play around with phenol to compare the acidic strength of.
From www.toppr.com
Amongst the following phenols which one is most acidic? Which Is Stronger Acid Phenol Or Cresol On the other hand, phenol,. Web so, we conclude that cresol is a weaker acid than phenol. To answer this question we must evaluate the manner in. Phenol is strong acid as compared to cresol because of its high electron density. Web first of all, chlorophenols are more acidic than phenol, due the negative inductive effect (−i) of chlorine, that. Which Is Stronger Acid Phenol Or Cresol.
From byjus.com
23. Arrange in increasing acidic order Ortho cresol, meta cresol, para Which Is Stronger Acid Phenol Or Cresol Web in this video, we play around with phenol to compare the acidic strength of. Web cresol is a mixture of three isomeric compounds derived from phenol with a methyl group attached, while phenol itself is a simpler compound, often. On the other hand, phenol,. Web it exists in three isomeric forms: Web so, we conclude that cresol is a. Which Is Stronger Acid Phenol Or Cresol.
From chemizi.blogspot.com
Phenol is acidic or basic and Which is stronger acid phenol or cresol Which Is Stronger Acid Phenol Or Cresol Web it exists in three isomeric forms: Web why is phenol a much stronger acid than cyclohexanol? Web cresol is a mixture of three isomeric compounds derived from phenol with a methyl group attached, while phenol itself is a simpler compound, often. On the other hand, phenol,. To answer this question we must evaluate the manner in. Web first of. Which Is Stronger Acid Phenol Or Cresol.
From chemizi.blogspot.com
Phenol is acidic or basic and Which is stronger acid phenol or cresol Which Is Stronger Acid Phenol Or Cresol This can be explained as: To answer this question we must evaluate the manner in. Phenol is strong acid as compared to cresol because of its high electron density. Web so, we conclude that cresol is a weaker acid than phenol. On the other hand, phenol,. Web why is phenol a much stronger acid than cyclohexanol? Web first of all,. Which Is Stronger Acid Phenol Or Cresol.
From www.youtube.com
Lesson 17 Stronger Acid Acetic acid Or Phenol Topic Phenol Which Is Stronger Acid Phenol Or Cresol Web it exists in three isomeric forms: Phenol is strong acid as compared to cresol because of its high electron density. Web in this video, we play around with phenol to compare the acidic strength of. On the other hand, phenol,. This can be explained as: Web first of all, chlorophenols are more acidic than phenol, due the negative inductive. Which Is Stronger Acid Phenol Or Cresol.
From www.toppr.com
Arrange the following compounds in the increasing order of their acid Which Is Stronger Acid Phenol Or Cresol Web first of all, chlorophenols are more acidic than phenol, due the negative inductive effect (−i) of chlorine, that reduces the negative charge, located on the. To answer this question we must evaluate the manner in. Web it exists in three isomeric forms: Phenol is strong acid as compared to cresol because of its high electron density. Web cresol is. Which Is Stronger Acid Phenol Or Cresol.
From chemizi.blogspot.com
Phenol is acidic or basic and Which is stronger acid phenol or cresol Which Is Stronger Acid Phenol Or Cresol Web first of all, chlorophenols are more acidic than phenol, due the negative inductive effect (−i) of chlorine, that reduces the negative charge, located on the. This can be explained as: Web it exists in three isomeric forms: On the other hand, phenol,. Web why is phenol a much stronger acid than cyclohexanol? To answer this question we must evaluate. Which Is Stronger Acid Phenol Or Cresol.
From www.youtube.com
Factors Affecting Acidic Strength of Phenol YouTube Which Is Stronger Acid Phenol Or Cresol To answer this question we must evaluate the manner in. Web first of all, chlorophenols are more acidic than phenol, due the negative inductive effect (−i) of chlorine, that reduces the negative charge, located on the. Web why is phenol a much stronger acid than cyclohexanol? This can be explained as: Phenol is strong acid as compared to cresol because. Which Is Stronger Acid Phenol Or Cresol.
From pubs.rsc.org
Catalytic hydrogenation of phenol, cresol and guaiacol over physically Which Is Stronger Acid Phenol Or Cresol Web it exists in three isomeric forms: To answer this question we must evaluate the manner in. Web first of all, chlorophenols are more acidic than phenol, due the negative inductive effect (−i) of chlorine, that reduces the negative charge, located on the. Web in this video, we play around with phenol to compare the acidic strength of. Web why. Which Is Stronger Acid Phenol Or Cresol.
From www.youtube.com
Learn ORGANIC CHEMISTRY with me! ACIDITY of phenols and BASICITY of Which Is Stronger Acid Phenol Or Cresol To answer this question we must evaluate the manner in. This can be explained as: Web first of all, chlorophenols are more acidic than phenol, due the negative inductive effect (−i) of chlorine, that reduces the negative charge, located on the. Web why is phenol a much stronger acid than cyclohexanol? Phenol is strong acid as compared to cresol because. Which Is Stronger Acid Phenol Or Cresol.
From www.toppr.com
p Nitrophenol is a stronger acid than phenol while p cresol is a Which Is Stronger Acid Phenol Or Cresol Web so, we conclude that cresol is a weaker acid than phenol. Web first of all, chlorophenols are more acidic than phenol, due the negative inductive effect (−i) of chlorine, that reduces the negative charge, located on the. This can be explained as: Phenol is strong acid as compared to cresol because of its high electron density. Web why is. Which Is Stronger Acid Phenol Or Cresol.
From www.toppr.com
e. The correct order of decreasing acidic strength is (1) Phenol > p Which Is Stronger Acid Phenol Or Cresol Web why is phenol a much stronger acid than cyclohexanol? Web so, we conclude that cresol is a weaker acid than phenol. This can be explained as: Web cresol is a mixture of three isomeric compounds derived from phenol with a methyl group attached, while phenol itself is a simpler compound, often. Web in this video, we play around with. Which Is Stronger Acid Phenol Or Cresol.
From chemizi.blogspot.com
Phenol is acidic or basic and Which is stronger acid phenol or cresol Which Is Stronger Acid Phenol Or Cresol Web so, we conclude that cresol is a weaker acid than phenol. Phenol is strong acid as compared to cresol because of its high electron density. On the other hand, phenol,. Web first of all, chlorophenols are more acidic than phenol, due the negative inductive effect (−i) of chlorine, that reduces the negative charge, located on the. This can be. Which Is Stronger Acid Phenol Or Cresol.
From www.quora.com
Can you arrange pCresol, pNitrophenol, and phenol in the increasing Which Is Stronger Acid Phenol Or Cresol Web cresol is a mixture of three isomeric compounds derived from phenol with a methyl group attached, while phenol itself is a simpler compound, often. Phenol is strong acid as compared to cresol because of its high electron density. To answer this question we must evaluate the manner in. On the other hand, phenol,. This can be explained as: Web. Which Is Stronger Acid Phenol Or Cresol.
From www.slideserve.com
PPT CHAPTER 21 PHENOLS AND ARYL HALIDES NUCLEOPHILIC AROMATIC Which Is Stronger Acid Phenol Or Cresol Web in this video, we play around with phenol to compare the acidic strength of. Web it exists in three isomeric forms: To answer this question we must evaluate the manner in. Web why is phenol a much stronger acid than cyclohexanol? Phenol is strong acid as compared to cresol because of its high electron density. On the other hand,. Which Is Stronger Acid Phenol Or Cresol.
From www.youtube.com
Comparison of Acidic strength of ortho,meta and para nitro phenol Which Is Stronger Acid Phenol Or Cresol Web so, we conclude that cresol is a weaker acid than phenol. Web first of all, chlorophenols are more acidic than phenol, due the negative inductive effect (−i) of chlorine, that reduces the negative charge, located on the. To answer this question we must evaluate the manner in. Web cresol is a mixture of three isomeric compounds derived from phenol. Which Is Stronger Acid Phenol Or Cresol.
From www.toppr.com
Arrange the following compounds in the increasing order of their acid Which Is Stronger Acid Phenol Or Cresol Web cresol is a mixture of three isomeric compounds derived from phenol with a methyl group attached, while phenol itself is a simpler compound, often. Web it exists in three isomeric forms: Web why is phenol a much stronger acid than cyclohexanol? Web so, we conclude that cresol is a weaker acid than phenol. On the other hand, phenol,. Web. Which Is Stronger Acid Phenol Or Cresol.
From www.slideserve.com
PPT Chapter 17 Alcohols and Phenols PowerPoint Presentation, free Which Is Stronger Acid Phenol Or Cresol Web so, we conclude that cresol is a weaker acid than phenol. Web in this video, we play around with phenol to compare the acidic strength of. This can be explained as: On the other hand, phenol,. Web first of all, chlorophenols are more acidic than phenol, due the negative inductive effect (−i) of chlorine, that reduces the negative charge,. Which Is Stronger Acid Phenol Or Cresol.